Effect of Various Factors on the Brazed Joint Properties in Al Brazing Technology
Article information
Abstract
Last few decades have seen a rapid increase in the fabrication and characterization of Al alloys for automobiles, heat exchangers and aerospace industries. Aluminium alloys are popular because of their high specific strength, light weight, excellent wear and high oxidation resistance. The development of aluminium alloys in these applications makes their study and research of utmost importance. Brazing is applied to the aluminium alloys for joining various aluminium parts together in most of the industrial applications. Various parameters affect the joining process of these aluminium alloys. In this article, various types of processing parameters have been discussed, and special attention has been given to the category of aluminium brazing alloys. The article reviews on the various parameters that affect the brazing property in various scientific and technological applications.
1. Introduction
The brazing refers to the joining process of heating a joint to the liquidus temperature of the used filler metal over 450 °C, and below the solidus temperature of the base material. During this process, the base metals are not affected due to a lower brazing temperature than their melting points1,2). It should be also noted that brazing temperature is always lower than welding temperature. Though brazing is similar to the soldering procedure yet the temperatures involved are even lesser than in brazing process, i.e. <450 °C. However, the welding temperature of a filler metal may be significantly higher than the melting point of the base material3,4). Brazing provides a metallurgical bonding between the contact surfaces and the filler metal without melting the base metal3-5). There are various types of brazing processes such as dip brazing, induction brazing, laser brazing, resistance brazing, furnace brazing, and oxyacetylene flame brazing6-11). A summary of different types of joining processes in metallurgy is described in Fig. 1.
A major advantage of brazing is that we can join dissimilar metals or ceramics with full perfection. There are various examples reported in literature where ceramic to metal joints are successfully brazed and investigated12-15). Direct or active metal brazing has been investigated where an active element like Ti or Zr is used in conjunction with the filler metal to wet the contacting surfaces. As a consequence, the need for conducting coating is minimized12).
2. Experimental steps in brazing
Brazing process generally composed of a series of steps depending on the joint to be brazed. The surface to be joined should be properly cleaned to avoid any defect in the brazed joint. The number of steps can be as follows:
Surface preparation
Proper fit and clearance
Use of suitable flux (NOCOLOK flux etc.)
Fixing the two parts to be joined
Heating the parts to the particular brazing temperature
Cleaning of the joint to avoid the residues, if any
3. Brazing parameters
Brazing parameters play an important role in the brazed joint characteristics. All the brazing joint parameters should be dealt with great care to produce a fruitful joint. Some of the important brazing parameters can be classified as follows:
3.1 Brazing temperature
Brazing temperature is the most widely investigated parameter. Temperature has a strong effect on the final brazed strength and the corrosion properties. A high temperature can change the brazed joint morphology and even may cause softening, cracks generation, ultimately leading to the joint failure. For example, in the active metal brazing of Al2O3/Cu joint with Ag-Cu-Zr-Sn filler, low brazing temperature is usually beneficial to maintain the superior bonding strength16). It is also reported that the interface thickness rises with the increase in temperature of silicon nitride joints using Ag-Cu-Ti-Mo filler metal under vacuum17). Yang et. al. found that different types of reaction products are formed while brazing Al2O3/Ti-6Al-4V with Ag-Cu-Ti-B filler. Different reaction products (i.e., TiCu, Ti(Cu, Al), Ti2Cu and Ti2(Cu, Al)) have their own growing speed towards the interface; which is a function of the brazing temperature, brazing duration and additive content, affect the overall joint strength directly18). Jiang et. al. have found that the brazing of stainless steel (304 grade) to fins with nickel based filler, the strength is increased with brazing temperature. The microstructures in brazed joints are changed with brazing temperature and brittle phases are found to decline with the increase in brazing temperature19). For aluminium based applications, low melting point braze fillers are generally used in the literature. For example, the popular Al-Si filler has been consistently researched and developed. In a recent study, Sharma et. al. incorporated ZrO2 nanoparticles in Al-Si-Cu filler to obtain improved joint properties as well as low melting point of the filler20).
3.2 Interfacial/multilayers at the interface
The formation of various interaction layers affects the brazed joint strength. It has been generally observed that interaction layers containing CuTi and Cu2Ti are formed in Ti-6Al-4V/ZrO2 using an Ag-Cu-Sn-Ti filler 12,21-22). It has been already discussed that various multilayers or reaction products form at different temperatures. Generally, Cu2Ti forms at low brazing temperature and increases at high temperature, thus imparting highest hardness value23). Therefore, a control of these reaction layers is important for achieving the best set of joint properties. A smaller thickness of these layers or IMCs corresponds to a higher brazed joint strength. An increase in the brazing temperature causes an increase in IMC thickness and vice versa24).
3.3 Wettability
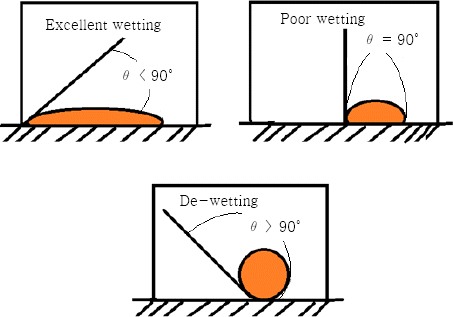
One of the very important criteria for any metal to be used as a filler in the brazing process is that it should wet and spread well over the contacting surfaces. Wetting can be defined as the ability of the molten filler metal to spread uniformly onto the surface of a metal after reflow procedure, and it should make a perfect bond with the base metal25). Figure 2.3 shows examples of wetting and de-wetting phenomena25).
Generally wetting depends on the wetting angle (θ) as shown in Figure 2. The wettability is an important factor in brazing applications. The contact surfaces to be joined should be wet enough to make a bond and avoid any faulty joining. The addition of Sn, and Ti in Al-Si alloy improves the brazeability by increasing the fluidity of the filler alloy26-27). However, a high amount of Sn is not desirable as it may increase pitting and can be deleterious for the joints26). Magnesium is also an important element that is used to increase the wetting in some operations. It provides additional strengthening to the alloy and improves work-hardening rate in multiple brazing filler operations. Magnesium containing metal is assumed as candidate replacement for high strength alloys, or steel, primarily in view of the excellent elongation, toughness, as well as better working characteristics28-30).
The wetting properties provided by the magnesium are generally due to the reaction of Mg with oxygen or moisture impurities as given by the following equations 31):
In the case of ceramic-metal brazing, wettability is a crucial factor due to poor wetting properties of engineering ceramics. It has been observed that the poor wettability of ceramic can be improved by using alloys such as modifiers and wetting promoters32). Various grain refiners such as, Ti, B, Sr, Na, Ca, P, Sb, Be, Ni, rare earth elements, oxides, etc. in brazing fillers are also added to increase the wetting and brazeability, and improve the quality of brazed joint and to produce minimum cracks and pores which may arise due to the non-uniform microstructures in Al-Si alloys33-39).
3.4 Manufacturing route
Various changes in the processing routes or employing an additional/sequential step may also reduce the cost and time. This can also affect the brazing microstructure as well as strength considerably. For example, the brazing of the extra pure Ti/Al2O3 joint brazed by the introduction of titanium hydride, the need for additional steps like metallization, electrodeposition and heating steps to the desired brazing temperature may be eliminated40).
3.5 Atmosphere
Brazing in a vacuum is relatively economical method to provide a controlled brazing environment. It is an excellent way of protecting the workpiece from oxidizing agents as well as foreign impurities. The vacuum level generally used for this type brazing falls in the range ≈ 10-3 to 10-5 mbar40). Other commonly used atmospheres are nitrogen, hydrogen, argon, etc. It has been reported that the brazing under controlled nitrogen atmosphere reduces the amount of flux required (NOCOLOK flux) 41-42). Reducing atmospheres too prevent the formation of surface oxides and reducing the metal oxides during brazing1).
3.6 Overlapped width
It has been reported that the shear strength of brazed joints is highly dependent on the lap width to a greater extent. The brazed shear strength of the joints increases with the decrease in the lap width. Similar results are observed when the lap joint of a highly pure titanium plate to a low-carbon steel plate is obtained under a vacuum furnace using silver-based filler23). It is suggested to employ a lap width up to a maximum of thrice of the thickness of the base metal for a superior brazed joint strength23,43).
3.7 Agitation
The effect of mechanical vibration also affects the strength of the brazed joint and the joining process. It has been reported that with an increase in vibration frequency from 0 to 400 Hz (and amplitude of 20 µm), the shear strength increases appreciably during induction brazing44).
3.8 Brazing duration
When the vacuum brazing of Ti-Al-based alloy to 40Cr steel using Ag-Cu-Zn filler is performed, it is observed that a change in brazing duration causes a change in brazing strength as well as a change in the joint microstructure and vice versa45). The brazing duration combined with the brazing time also affects the joint properties to a greater extent18,45).
3.9 Effect of different filler materials.
Many different varieties of filler alloys are available in brazing applications. Some of the brazing filler materials and their effects are tabulated below31):
4. Summary of the effect of different brazing parameters
Brazing is a technologically efficient process for the joining of the metal, nonmetals and ceramics to one another. Brazing has a wide promising future in automobiles, automotive, and aerospace industrial applications. More specialized methods are expected to emerge in future due to a vast development in the automotive and aerospace vehicles. Therefore, this paper throws a light on the effect of various parameters on the various brazing techniques and materials for a better understanding of the overall technological aspects. There are following conclusions that can be drawn from the present review.
Temperature has a vital role in the brazing process. Increase in temperature improves the brazeability, however, it should not be too high to cause enough formation of IMCs that decreases the joint strength.
Brazing duration should be enough to cause a proper reaction between the joints otherwise the wetting will be poor.
Formation of interfacial layers is crucial for the brazing technology. Therefore, a strict control of IMCs is required via temperature or time optimization.
Agitation during the brazing process improves the shear strength of the joint by eliminating the impurities and cracks.
A proper lap width should be selected to exercise the maximum benefit of the brazed strength.
Manufacturing route also affects the brazing process. Brazing is different in vacuum and in inert atmospheres depending on the materials to be joined.
Wetting of the surfaces is also an important factor. The braze filler should have proper wetting, that is further dependent on the number of additional elements present in the filler. Therefore, a proper brazing filler with a proper amount of the additive is required.
Acknowledgements
This work was supported by the Energy Efficiency & Resources Core Technology Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) granted financial resource from the Ministry of Trade, Industry & Energy, Republic of Korea (No. 20142020104380).