3.1 Magnetic pulse welding
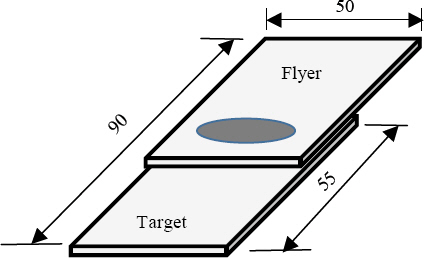
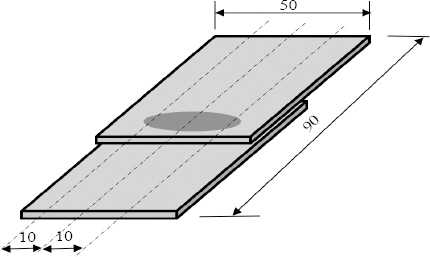
A weldment specimen as shown in
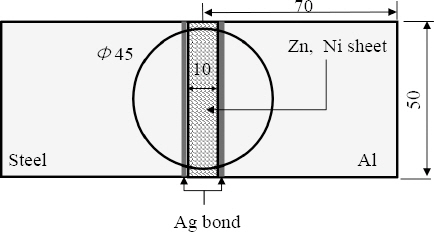
Fig. 7 was fabricated through magnetic pulse welding process. The specimen shown in the figure is its flat image that was cut to the specification for the joining part corrosion test. The welding condition was 13kV of applied voltage, the distance between the flyer material and the target material was 2mm, and the heat input was about 40kJ. Heat input can be expressed as energy as below.
Fig.┬Ā7
Corrosion test specimens after MPW under 40kJ heat input

Here, E is the junction energy (J), C is the capacitor bank capacity (uF), V is the applied voltage (V), and B is the number of capacitor banks used.
Three types of specimens were fabricated as follows: non-coated steel sheet and Al1050 (Al-Fe), Ni-coated steel sheet and Al1050 (Al-Ni-Fe), and Zn-coated steel sheet and Al1050 (Al-Zn-Fe).
3.2 Electrochemical corrosion behavior test
Fig. 8 shows the Tafel curve of the specimen reproducing the dissimilar joining part. The corrosion potential (E-corrosion) is about -0.9V for the Al-Zn-Fe combination, about -0.85V for the Al-Fe combination, and about -0.8V for the Al-Ni-Fe combination, making the Al-Ni-Fe combination the highest and the Al-Zn-Fe combination the lowest. This is considered to be due to the fact that Ni is a noble metal compared to Zn and Fe, and has better electrochemical properties.
Fig.┬Ā8
Tafel curves for dissimilar joints

In addition, from the viewpoint of corrosion current, Ni is the highest, followed by Zn and Fe. These findings indicate that until corrosion begins, the corrosion resistance of the Ni coating layer is the most excellent, but once corrosion starts, the most corrosion is likely to occur in the case where Ni is used as the coating layer, and the amount of corrosion decreases when there is no coating layer.
3.3 Corrosion resistance of magnetic pulse weldment
Fig. 9 shows the results of the tensile shear test after the corrosion test for each material combination of weldment specimen.
Fig. 9 (a) is the result of tensile shear test on the immersed specimen. In the case of Al-Zn-Fe, tensile shear deterioration occurred from the beginning of the corrosion test because the joining part was detached due to the early corrosion of the joining part as the corrosion test proceeded. (
Fig. 10). In the case of the Al-Fe and Al-Ni-Fe combinations, the fracture did not occur in the joining part, but the tensile shear was lowered over time. Therefore, it is thought that the tensile shear strength was lowered as the Al base material corroded.
Fig.┬Ā9
Tensile test results of MPW joint after corrosion test (a) Immersion (b) Atmospheric exposure
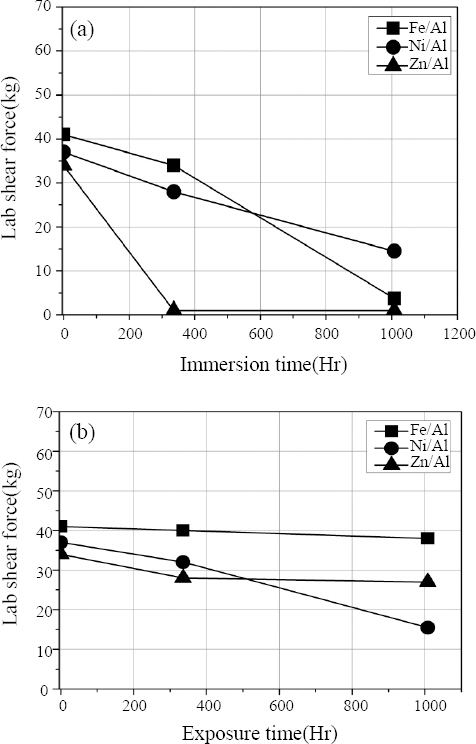
Fig.┬Ā10
Tensile test specimens after 1008hr corrosion test of MPW joint

In the case of Al-Fe combination, since there was no coating, it is thought that the tensile shear strength gradually decreased as corrosion progressed, and the case of Al-Ni-Fe showed to have maintained the highest tensile shear strength and the reason for this is that as can be seen from the results of the electrochemical corrosion test, this combination has higher corrosion potential than other combinations, leading to not much progress of the corrosion. Overall, welding tensile shear strength showed a sharp decrease in the immersion specimens compared to the atmospheric exposure specimen, and this is thought to be due to the fact that the contact area and the amount of contact with the corrosive solution were higher in the case of immersion specimen. In addition, it is considered that no treatment on the side of the specimen may have led to the acceleration of the corrosion, but this needs to be verified through additional experiment.
Fig. 9 (b) is a result of the tensile shear test of the specimen exposed to the atmosphere and shows a different pattern from the immersion specimen. As shown in
Fig. 10, after 1008 hours of corrosion test, in the case of Al-Fe combination, fracture occurred in the Al base and in the case of Al-Zn-Fe, joining part was detached or fracture occurred in the Zn-coated steel sheet base and in the case of Al-Ni-Fe, fracture occurred in the Ni-coated steel sheet base. In the case of Al-Fe and Al-Zn-Fe combinations, there was no change in tensile shear strength after 1008 hours, but in the case of Al- Ni-Fe, tensile shear strength was low after 1008 hours, which means that the thickness of Ni-coated steel sheet is 0.2mm, thinner than other materials, indicating faster progress of corrosion. Furthermore, since there was no coating treatment on the side in the specimen fabrication, leading to the effect of large potential difference between Ni and Fe showing faster, thereby accelerating the corrosion to proceed.
In the case of the Al-Zn-Fe combination, the thickness of the Zn-coated steel sheet is thinner than that of the Al1050 material, and the length is also short, and it is thought that the corrosion effect of Zn-coated steel sheet was large.
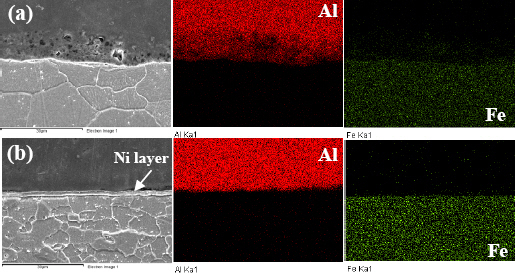
Fig. 11 shows the results of analyzing the cross section of the specimen after the corrosion test. In the case of the Al-Fe combination, there is a trace of corrosion on the Al side, and the distribution of Al is also blurred in the EDX analysis results, indicating that corrosion has occurred. On the other hand, in the case of the Al-Ni- Fe combination, there are some traces of corrosion on the Al side, but unlike the case of the Al-Fe combination, there is no remarkable corrosion pattern.
Fig.┬Ā11
SEM/EDX analysis results of MPW joints after immersion corrosion test. (a) Fe - 336hr (b) Ni - 1008hr
In the case of fusion welding, the weldments are fused and then solidified provoking a structural change , resulting in a potential difference, and thus a galvanic corrosion phenomenon occurs in which corrosion proceeds first. In the case of magnetic pulse welding, there is no fusion, so the joining part is about several to several tens of ┬Ąm, and there is almost no structural change. Therefore, in the case of magnetic pulse welding, corrosion occurs due to the corrosion potential difference between Al and Fe itself, and the Al side with a low corrosion potential is thought to be corroded first.
According to Kang et al.
10), a similar tendency was found in the spot resistance weldment salt spray test, but the spot weldment tensile shear strength showed greater reduction in the GA steel sheet than that of CR steel sheet. Therefore, in the case of coated steel sheets, corrosion resistance is different depending on the corrosion environment, so it is necessary to select a coating material suitable for the environment of use, and it is difficult to conclude that the case where a coating layer is present in the joining part is always advantageous for corrosion resistance.











 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print