A Review of the Brazeability of Low-Temperature and Nano-Reinforced Al-Based Brazing Filler Metals
Article information
Abstract
Aluminum and its alloys are some of the most widely used materials for the brazing of parts in aerospace and automotive industries due to their excellent physical characteristics, e.g., good ductility, high strength-to-weight ratio, light weight, good corrosion and oxidation resistance, satisfactory malleability and formability, and excellent thermal and electrical conductivity. Especially during casting operations, high strength and machinability of Al-based alloys are of utmost concern. The quality and strength of casting products are affected by porosity generated due to the dissolution of gases and the effects of modifiers. Thus, unstable properties of Al alloys arising due to such influences should to be controlled by reinforcing suitable elements. Compared to other materials, brazing on Al requires higher control precision as the difference between the melting points of the base metal and the filler remain low even after the addition of melting point suppressants, such as Si. This necessitates the development of novel low-temperature Al-based filler metals. Moreover, the mechanical properties of Al-based alloys can be enhanced by introducing nanoparticles within them to reduce the thickness of Si-needles and IMCs (intermetallic compound) within the Al matrix, thereby refining the nanostructure.
1. Introduction
Aluminum and its alloys are the most preferred materials used in many industrial applications including electronics packaging, automotive, and aerospace industries because of their excellent electrical, thermal and mechanical properties with good corrosion resistance and excellent strength to weight ratio. Therefore, the most important concern is the bonding technique used in industries for joining aluminum and its alloys due to the dependency of higher reliability of Al on its joining technology. Hence, to accomplish a good joint, the excellent properties of Al and its alloys are essential1-3). In Al industries, adhesive bonding, mechanical joining, brazing and welding are the widely used joining techniques. Among all the available methods for Al bonding, brazing is a simple and efficient method to fabricate a quality joint. Brazing is an ancient (5000 years old) joining technique, which requires a filler metal to be melted between two or more materials to fabricate a strong metallic bond. Once the brazing filler is molten and spread across the whole joint area, the assembly is left to cool and a strong metallic joint is formed after the solidification of the filler metal. The principal characteristics of this process, which creates a difference from other joining techniques, is the filler metal used to fabricate the joints between the materials to be joined. The essential requirement of the filler is to wet the entire joint area properly4) and must have a lower liquidus temperature than the base materials. If the liquidus of the filler metal is higher than the base metals, then it leads to melting of the base materials during joint formation and the process would be referred to as a kind of welding. Moreover, to differentiate brazing from the soldering process, a lower limit of 450 °C was imposed on the liquidus of the brazing fillers. More interestingly, the brazing technique has the capability to join not only the dissimilar metals (metal to metal), but also to fabricate an excellent dissimilar joint between entirely different classes of materials (metal to ceramics). Besides, the joints formed by using this technique often elicit minimal evolution in the composition and microstructure of the base materials, making brazing an indispensable technique in modern manufacturing. The increasing use of brazed components in the severe environment necessitates the development of the novel brazing filler metals where the traditional fillers can no longer function properly5,6).
Generally, for Al brazing conventional Al filler metal, based on Al-12 wt% Si, is employed in brazing industries due to the ability of Si to reduce shrinkage defects and enhance the fluidity of molten filler7). However, the melting points of conventional Al-12 wt% Si filler (577 °C) and Al are very close to each other. This leads to the localized melting and deterioration of base material after joint formation, which can degrade the joint by crack formation and leads to joint failure8). Additionally, the formation of brittle Si particles also imposes a serious issue on joint performance which necessitates the development of new filler with improved mechanical properties and lower melting point for brazing of Al alloys. Researchers are actively working on the development of novel filler metals for Al brazing and also reported the filler metal with comparatively low melting point. Humpston et al.9) developed a brazing filler containing Al-20 wt% Cu-2 wt% Ni-5 wt% Si having a melting temperature of approximately 525.85 °C that was for bonding Al-alloys. Although, the Cu addition considerably enhanced the joint strength, however, a higher amount of Cu addition has an adverse effect on the joint strength10). Suzuki et al.11) reported a low-temperature brazing filler comprising of Al-4.2 wt% Si-40 wt% Zn-Sn with a melting temperature of approximately 535 °C. Moreover, Kayamoto et al.12) developed an Al-Ge-Si-Mg containing brazing filler, with mass percentage of elements containing 15-45 % Ge, 12 % Si, 0.6-0.9 % Mg, and Al was balance, to fabricate a sound and high strength joint for Al-alloys. The melting point of the joint was reported to be around 565 °C.
In the brazing process, the formation of intermetallic compounds (IMCs) is a serious issue because it can result in the development of pores and cracks at the fabricated brazed joint. Hence, it is needed to suppress the growth of IMCs and refine the alloyed microstructure. Recently, nanoparticle reinforced brazing fillers have been used to get a well-dispersed and refined microstructure in order to achieve enhanced brazeability and mechanical properties because of the higher surface energy of nanosized materials13-15). This manuscript reviews the effect of nanoparticle reinforced Al-based brazing filler on microstructure and brazeability of aluminum alloys.
2. Material joining methods
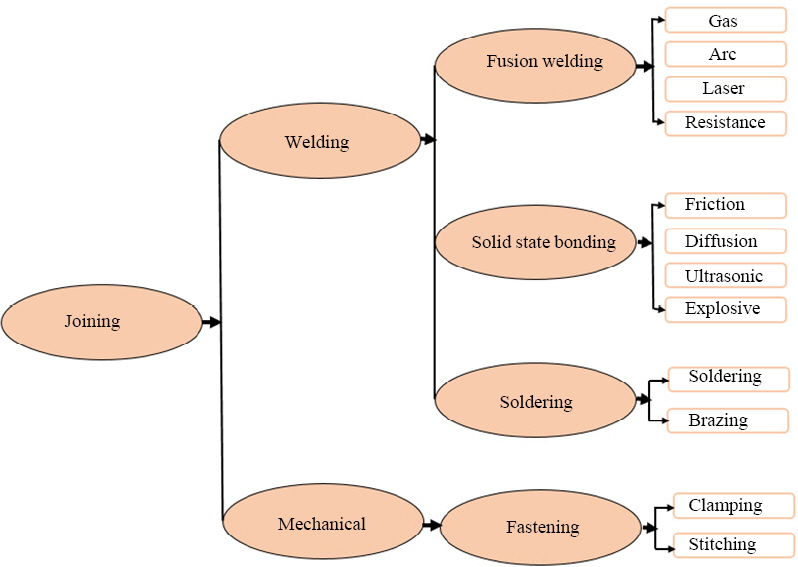
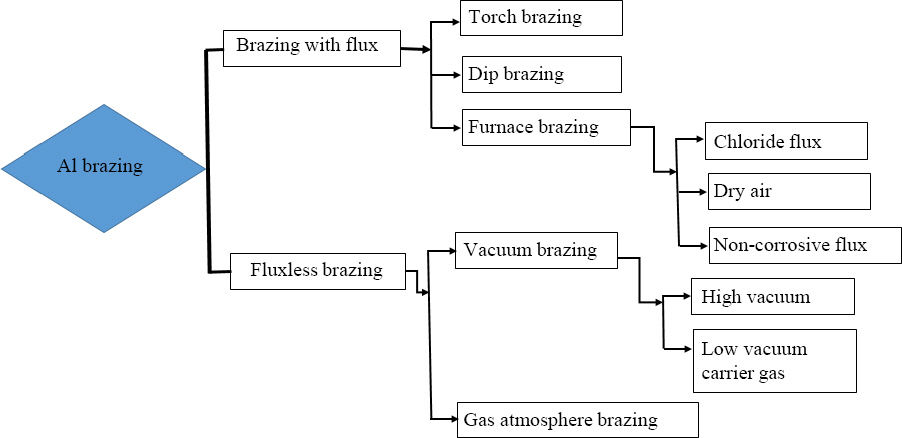
The manufacturing of big parts in heavy industries such as wing sections and fuselage of aircraft or automobile bodies needs a complex and large mold, which led to increasing the cost of the process. However, such large and complex parts can be produced by employing different joining techniques by making an assembly of small components. For joining of materials, various joining techniques are available as shown in Fig. 1. For instance, mechanical fastening is mainly employed in industries to assemble the large composite structure using bolts and rivets. However, the major drawback of using mechanical fastening is associated with the stress concentration taking place because of the holes in the substrate. Moreover, it led to adding extra weight to the assembly16). Fusion welding include melting of the parent metals which leads to influence the microstructure of the fusion zone. Whereas, in brazing, only filler metal gets melted, hence parent materials experience negligible changes in microstructure during the process, which makes brazing a suitable process for various engineering applications.
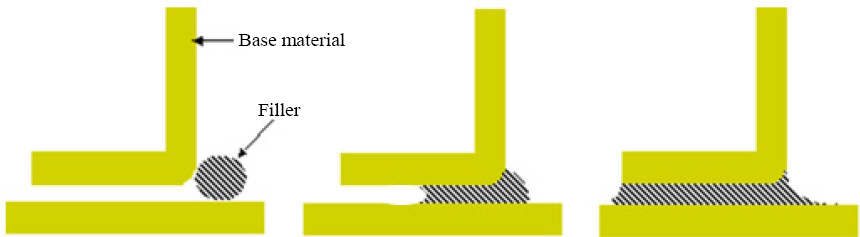
Brazing is a process that involves a joint between two similar or dissimilar materials (known as parent materials) with the help of filler metal, having the liquidus temperature above 450 °C but lower than that of the parent materials. The filler used in this joining process varies according to the parent materials that need to be joined, but essentially it must be capable of wetting the surface of parent materials and should possess a lower liquidus temperature than the base materials17).
Brazing technology provides a number of additional features as compared to the other available joining processes including welding, adhesives, and fastening. Hence, it can be a very useful and versatile process for the effective joining of materials. The first and foremost advantage of brazing and one of the most favorable reasons for its use in advanced engineering is the ability of this process to fabricate a sound joint between entirely different kinds of materials (such as metal to ceramics) and that too with the minimal modification of the materials being joined. In contrast, the welded joint provides a comparatively strong joint. It predominantly needs a similar kind of base metals and due to intensive local heating thermal distortion becomes a general problem, which can be avoided by using furnace brazing due to uniform heating of the assembly. However, the operating temperature of brazed assemblies is lower than that of fusion welding and is often comparatively weaker. Commonly, the brazed joint has strength greater than the filler but lower than parent materials. Despite this, if the designing and joining process performed correctly, the fabricated brazed joint can also have sufficient strength than the base materials17).
3. Wettability and spreading behavior
In our previous investigations, the author with his colleagues reviewed the influence of different factors on the brazing process and concluded that the brazing filler should be able to wet and spread all over the joint surfaces18-20). Wetting and spreading behavior of a filler metal are important terms and provide information about the flow of filler in molten state. Generally, free- flowing brazing fillers are preferred over the one which moves more sluggishly because it can penetrate comparatively lesser joint clearance. Moreover, a free-flowing filler metal is also very useful in the brazing processes where pre-placement of filler within the joint is not possible. In contrast, too free-flowing nature of brazing filler can lead to the flow of filler away from the joint area rather than staying in it. Therefore, it may lead to the development of voids and eventually affect the joint performance. The balance of force of adhesion (interaction between the molten filler metal and surface of the substrate) and cohesive forces (interaction between the atoms of molten filler metal) are generally taken into consideration to get an idea of the wettability of a filler. To obtain a good wetting over the substrate surface the force of adhesion must be greater than the force of cohesion within the atoms of filler21).
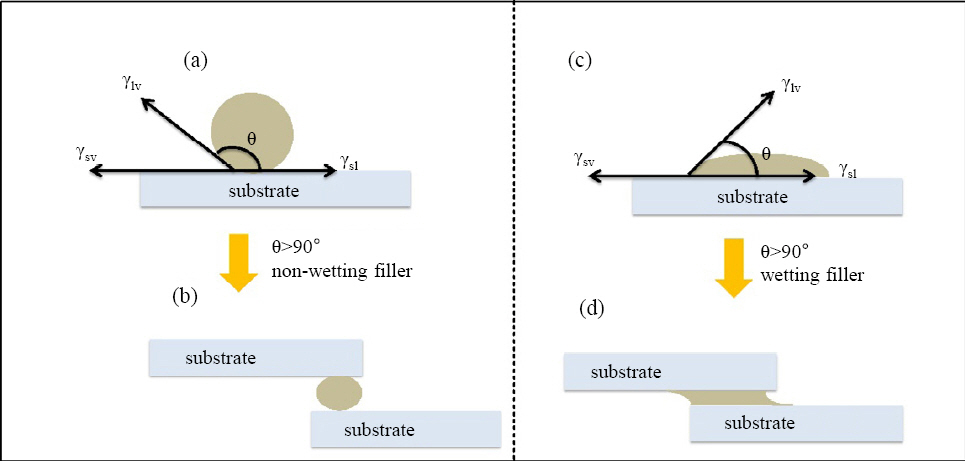
The wetting angle and the wettability of the filler are the outcomes of balance between forces of cohesion and adhesion as illustrated in Fig. 3. These forces can be explained in terms of surface energy, γlv and γsv liquid surface tension and surface free energy of the solid, γsl the interfacial energy between solid-liquid interface, respectively. According to the presented model, {γsv - γsl} is the driving force for wetting the surfaces. The horizontal component of γlv, i. e. γlv cosθ is responsible to the provide the balancing force for achieving equilibrium within the system. In 1805, Thomas Young presented an equation and proposed a relationship between γlv, γsv, and γsl as shown by equation 1.
Furthermore, to get an idea of the spreading behavior of filler metal on the surface of substrate, a spreading parameter S was introduced.
If S >0; high spreading occurs to minimize the surface energy.
If S <0; partial or poor wetting, the filler tends to form a spherical drop on the solid surface.
When the adhesive forces are higher than the cohesive forces, it tends to form a lower contact angle (θ < 90°) which balances Young’s equation. Hence, leads to good wettability and spreading of the filler metal across the joint surfaces. In contrast, if the cohesive forces are greater than the adhesive forces, it tends to form a contact angle greater than 90 ° (θ > 90°). Therefore, resulting in a non-wetting liquid. Therefore, it can be concluded that greater adhesive forces between molten filler and substrate surfaces correlate with lower wetting angles.
4. Base metal dissolution kinetics
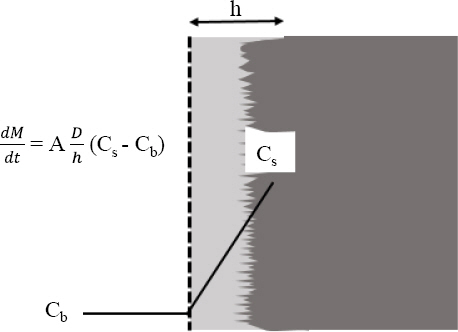
Noyes and Whitney developed the first transport-controlled dissolution model, according to this model the rate of dissolution is directly proportional to the bulk concentration and solubility difference, as given by equation 3.
Where M denotes the dissolved amount as a function of time, Cb and Cs represent bulk concentration and solubility, respectively. k is the dissolution rate constant. The proposed model was simple and seminal but failed in order to elucidate the physical meaning of dissolution rate constant about its dependence on transport properties of the solute. Later, Nernst and Bruner investigated this theory and improved it by introducing the stagnant diffusion layer concept, across this diffusion layer the solute from the solid surface diffuses to bulk solution and leads to forming a linear concentration profile. This investigation gives a simple and distinct meaning to dissolution rate constant k.
Where, D; diffusion coefficient, h and A is diffusion layer thickness and area of solid surface respectively.
The Nernst-Bruner equation is used to govern the base metal dissolution phenomenon. The dissolution of base metal is nearly related to the removal of oxide layer or machined layer on the bonding surface of parent metals, which is the key phenomenon for understanding the mechanism of bonding. It is reported to affect the migration and structure of the liquid-solid interface. Moreover, it is also reported that grain boundaries have the ability to dissolve base metal into the filler22). Hence, Nernst and Bruner proposed a modified equation as presented by equation 5.
5. Filler metals
Filler is the alloy (or elemental metal) used to fabricate a brazed joint. It is applied between the parent materials and has a lower melting point than the used base materials. During the brazing process the filler metal melted and the assembly is allowed to solidify, forming a brazed joint. The selection of appropriate filler metal is essential to fabricate an excellent brazing joint. Different factors affect the selection of suitable filler metal including;
The parent materials: the metallurgical and mechanical compatibility between the base metal and the filler.
The conditions during service: temperature and environment of operation, level, nature and type of mechanical loading, or the existence of any corrosive medium.
Design of joint: suitable flowing nature for the joint clearance.
Brazing operation: particular fillers are unsuitable for particular brazing operations (such as zinc-containing filler metals for vacuum brazing process).
Form of filler: foil, paste, wire etc.
Prior to the selection of filler metal, the parent materials and the operating environment of the fabricated assembly are generally fixed. However, for ease of use, the brazing filler metal should comprise the lowest melting point and should have the most free-flowing nature that fulfils the requirements of the application.
6. Low-temperature Al-based brazing fillers
The most significant limitation for materials having low melting temperature such as Al-alloy, is that the filler metal must be able to melt before the parent materials, in other words, the filler must possess a lower melting point than the parent materials. Generally, the filler metals used for brazing are mostly based on the Al-Si alloy system, with Si suppressing the melting temperature of the filler to 580-630 °C range.
As compared to other materials, performing brazing on Al requires more precise control because even after the addition of melting point suppressing element Si, the difference between the melting point of base metal and filler are reported low (as small as 10 °C) which led to the narrow process windows. In industries until 1980, the brazing of Al alloys was not performed on a large scale, when acceptable control of temperature was achieved by furnace or chloride salt bath brazing with the help of fluxes having high corrosive residues that needed extensive treatment after brazing23). Vacuum brazing was very much expensive at that time for all but specialist aerospace applications and aluminum brazing in air is possible with some fluxes, these are corrosive such as FL10 and require high temperatures, nearly equal to the melting point of aluminum to be activated. However, effective and efficient heat exchangers are required for air conditioning systems because of the increasing demand for these systems in modern cars. Moreover, the need for a reduction in weight for these structures led to replacing the copper and brass with lightweight aluminum. Hence, for Al brazing, an improved method was needed and this led to the development of the Solvay brazing process. Interestingly, fluoroaluminate salt mixture is used as flux in this process. Which dissolves the layer of Al oxide, once heated to its melting range (565-572 °C, 5 °C below the melting point of Al-12 wt-%Si filler) and helps in preventing further oxidation. This flux system has no vacuum requirement instead a nitrogen atmosphere is required and leaves behind a non-corrosive residue. The advancement of this process and the development of this particular flux enables brazing of Al at an acceptable cost to permit the commercialization of Al heat exchangers. In the manufacturing of Al radiators and heat exchangers, the metallurgical bond took place between the filler metal and Al sheet during fabrication. With aluminum-silicon cladding covering one side or both sides of the assembly. The aluminum sheet is then assembled into shape and placed in a furnace to melt the outer cladding layer and form the bond24).
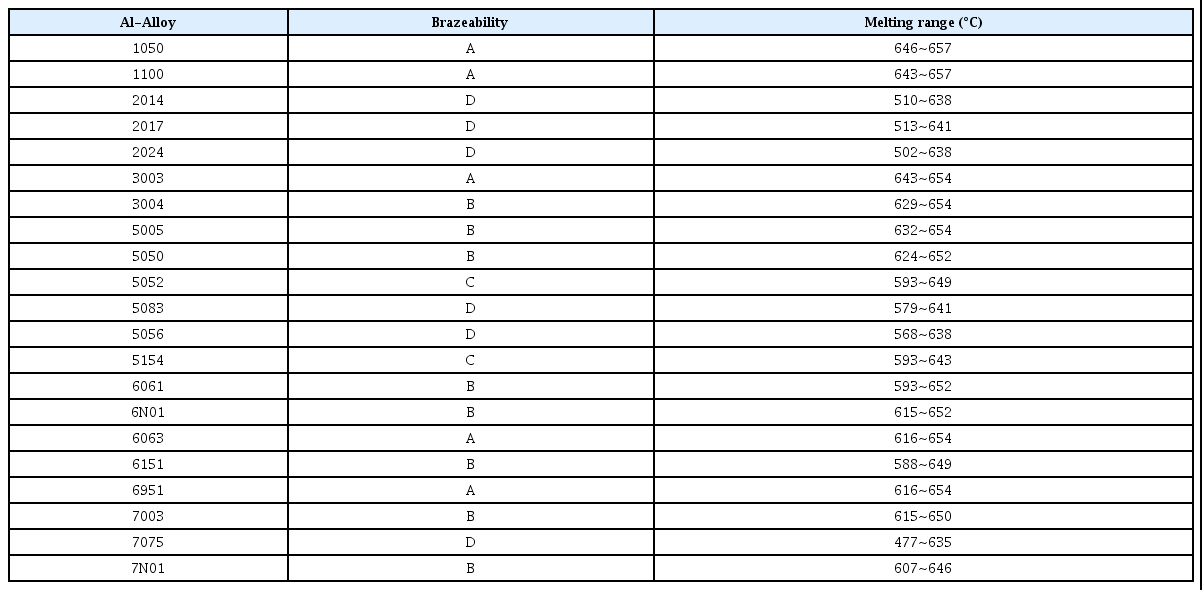
The widespread use of Al and its alloys especially in aerospace and automotive applications led to the development of Al-based filler metals. The high strength to weight ratio and reduction in the weight of Al alloys make them suitable for various applications as it helps in improving fuel efficiency and reducing the consumption of power. The combination of excellent properties of Al alloys such as high strength, good oxidation and corrosion resistance, workability, machinability, and better damping capacity makes it the highly used material after cast iron and steel25). The combination of these excellent properties of Al-alloys is dependent on the existence of additional elements, heat treatment, and cold working. To further improve the properties of Al-alloys, wide investigations have been performed by the addition of alloying elements, impurity addition, and by using modifiers etc.25-27). The additional alloying elements may cause the development of IMCs, hence these IMCs are the key elements in accessing the microstructure and mechanical properties of Al-alloys. Moreover, the poorly distribution of these specific IMCs within Al-matrix may lead to the failure of these alloys due to the intergranular and pitting corrosion27). Generally, the most commonly used alloying elements for Al are magnesium, silicon, copper, manganese and zinc. Al-Cu alloy systems are the widely used alloy system for aerospace applications. Because of these excellent properties and flexibility of addition of alloying elements, Al-alloys are one of the most investigated materials for academic as well as industrial research point of view. These alloying elements also have a significant influence on the brazeability of these alloys. Hence, the selection of alloying elements should be made based on the effectiveness, melting range and suitability for particular applications. The below Table 1, provides information about the brazeability and melting range of different Al-based alloys which are graded as A, B, C, and D based on the effectiveness and suitability of particular alloy for brazing application.
7. Effect of nanoparticle reinforcement on Al brazing fillers
Al and its alloys have wide industrial applications in electrical connectors, capacitor and condenser foil windings, and foil conductors in transformers. These assemblies consist of a large number of similar and dissimilar joints28). Preferably, in dissimilar joints, many challenges occurred in achieving a reliable joint because of the difference in chemical, physical and mechanical properties of the materials which lead to the formation of IMCs. The addition of nanoparticles has been reported as the controlling method for IMCs growth as well as interfacial morphology microstructure during bonding29,30). In such applications, the selection of appropriate filler metal with nanoparticle reinforcement is useful in order to fabricate a reliable joint with enhanced microstructure and mechanical properties. Among most of the ceramics for engineering applications, ZrO2 nanomaterials have very good mechanical strength at room temperature. Moreover, the coefficient of thermal expansion of ZrO2 is close to metals which makes it a suitable alloying element for various metals. The authors31) investigated the effect of ZrO2 nanoparticle reinforcement on the microstructure, mechanical properties and spreadability of Al-12Si-20Cu filler for Al alloy brazing by fabricating Al-12Si-20Cu with reinforcement of 0 to 0.1 wt % ZrO2 nanoparticles. At a concentration of 0.05 wt % ZrO2, the well-dispersed phases were observed in the matrix and further addition of ZrO2 reveals no effect on the microstructure. This can be attributed to the weakening in the microstructural refinement at 0.1 wt % ZrO2 segregation effect at an increased concentration of nanoparticles in the molten metal. Moreover, due to the refinement of microstructure at 0.05 wt % ZrO2, the microhardness value showed an increment of 13 % with a reduction of 2 °C in the liquidus temperature. Jung et al.32) performed experimentation to observe the effect of ZrO2 nanoparticles on interfacial microstructure and wettability of the Al-19Cu-11Si-2Sn filler for dissimilar brazing of Al to Cu. The filler was cast by using an induction furnace and processed in the shape of a ring for brazing. The refinement of Al-19Cu-11Si-2Sn brazing filler with Si and CuAl2 phases were observed with the reinforcement of 0.1 wt % ZrO2 nanoparticles, which considerably improved the mechanical properties. Moreover, a significant refinement in the Si needles and CuAl2 phases was reported after the addition of 0.1 wt % of ZrO2 nanoparticles. In the matrix, the ZrO2 nanoparticles act as the nucleation site and the growth of silicon particles would be controlled by neighboring nanoparticles of ZrO2. Choi et al.33) has also reported similar observations with the addition of Al2O3 particles to Al-12Si. Moreover, possibly the nanoparticles of ZrO2 may cling to the CuAl2 intermetallics and get absorbed there, resulting in the restriction of the growth of CuAl2 IMCs during the solidification process. Hence, the reinforcement of ZrO2 nanoparticles has a considerable effect on controlling the formation of Si and Cu intermetallic compounds in low-temperature Al-based fillers.
The authors34) studied the microstructure and brazeability of the 0.5 wt % SiC nanoparticle reinforced Al-9Si-20Cu alloy. The refinement of CuAl2 and Si was reported in Al-9Si-20Cu-0.5SiC composite microstructure. The spreading ratio was improved upto 88.4 % for Al-9Si-20Cu-0.5SiC composite as compared to Al-9Si- 20Cu alloy that exhibits 76.67 %. Moreover, 0.5 wt % SiC nanoparticle reinforcement results in improved ductility and tensile strength. Lan et al.35) studied the dispersion of SiC nanoparticles in the molten metal by ultrasonic non-linear effects and fabricated SiC nanoparticle reinforced AZ91D magnesium composite. The microstructure investigation revealed a uniform distribution of SiC nanoparticles within the magnesium matrix, although, within the matrix, few agglomerates were observed. Moreover, the authors reported the partial oxidation of SiC nanoparticles. The enhancement in the properties of Al-based composites can be attributed to the strengthening effects such as dislocations, substructures, and grain-boundaries (GBs) etc. Similarly, Wang et al.36) employed ultrasonic method to fabricate SiC nanoparticle reinforced magnesium matrix composites. The microstructure and mechanical investigation revealed the uniform and effective dispersion of SiC nanoparticles within the magnesium alloy with the help of ultrasonic vibration. Moreover, the magnesium composite with refined grains and improved mechanical properties were observed. The improved mechanical properties can be attributed to the strengthening effects and grain refinement caused by SiC nanoparticles.
8. Conclusion
Brazing technique has been widely used in different industrial and engineering applications since it was employed as a joining technique around 5,000 years ago. However, it is evident that brazing is still the key technology for joining of entirely different materials such as metal to ceramics. Fillers for the brazing process are adapted and modified as per the requirement for particular type of joints, and the continuous development of novel materials necessitates the development of new kind of filler metals. For Al, alloying elements play a significant role in deciding the final microstructure of the alloy. A slight modification of alloy microstructure by the reinforcement of nanoparticles lead to drastically change the ductility, strength, machinability, castability, brazing properties and energy efficiency in different industrial applications. Moreover, the silicon addition enhances the fluidity of Al alloys and hence, improves the castability of the alloys. However, the addition of high amount of silicon leads to the development of undesirable big Si needles in the hypereutectic alloy systems which results in the reduced strength of the alloy. The addition of Cu imparts strength to Al alloys due to the formation of CuAl2 IMCs. However, the formation of CuAl2 in huge amount is also problematic during brazing and structural applications. Interestingly, the nanoparticle reinforcement leads to refining the microstructure of the Al-alloys by reducing the thickness of Si needles and intermetallic compounds, which helps in the enhancement of bonding strength of the brazed joint. Moreover, the addition of AlN, SiC, and ZrO2 improves the spreading ration and reduce the liquidus temperature of the alloys.
Acknowledgement
This paper was supported by Korea Institute for Advancement of Technology(KIAT) grant funded by the Korea Government(MOTIE) (P0018010, 2022)